In February 2015, the FDA issued a ruling that is having a chilling effect on sales of laser phosphor projectors in the US. This ruling classified projectors containing lasers (RGB laser, laser phosphor and hybrid) in a much more restrictive way than the EU IEC ruling. The ramifications of this ruling were visible at Infocomm.

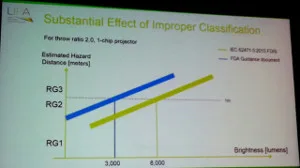
The bottom line is that laser-based projectors with an output only 3,000 lumens are now considered by the FDA to be in the Risk Group 3 category, which means a variance to operate the projector is needed along with special height requirements being needed and the potential to have a laser safety officer on-site. The IEC sets the bar to trigger these measures at 8,000 lumens (a much more reasonable level – CC). The effect of the FDA ruling is that essentially all laser-phosphor and RGB laser projectors are now Risk Group 3, which will have a very negative effect on their adoption in the US and other regions where FDA guidelines are followed.
Noticeably absent from Infocomm were the Chinese brands that are offering laser-phosphor projectors. They apparently did not want to exhibit as they don’t want to sell in the US with such a draconian FDA ruling. A 3,000 lumen laser-phosphor projector placed on a conference room table would now be considered in violation of the FDA ruling. This is the mainstream market, so the ruling is a big problem for projector makers.
At Infocomm, Barco was taking the ruling seriously. In their laser-phosphor retrofit demo, they placed the projector on a tower and made sure the beam was 8’4” (2.54M) above the floor so that no one could inadvertently look into the beam. Panasonic is marking its laser-phosphor projectors as class 3R laser products. And on the show floor, there were many other laser phosphor demos, but in almost all cases, the projectors were mounted high enough to comply with the ruling.
How did we get to this point? According to Greg Niven, who represented the Laser Illuminated Projector Association (LIPA) in a presentation given at SID in early June, the organization has had success in Europe (IEC) and Japan on regulations regarding laser and laser-phosphor projectors, but it has not been as successful in the US. Niven explained that the organization’s efforts have been focused on convincing regulatory agencies that a laser projector – whether powered by RGB lasers or a laser-phosphor engine – should be treated like lamp-based projectors, not a laser light show device or laser welding solutions. That may seem obvious, but it has taken tremendous effort and research to prove this.
The basis of the argument is that an RGB or laser phosphor projector expands the beam of light to fill a microdisplay. As a result, it is just like a projector powered by a conventional lamp source. Any light exiting the projector is beam expanded and diverging – not a tight, collimated laser beam.
Eye damage is based on power density, so small beams with high power are dangerous, but the same power over a big beam may not be harmful. That is the case for lamp-based and laser-based projectors so, LIPA says, they should be treated the same.
To drive home this point, LIPA funded a solid research project describing the architecture of projectors and the laser power density at various points in the system. LIPA also developed a new way of characterizing these projectors: Risk Groups. This has now been extended to projectors with interchangeable lenses as well. The table below shows the definitions of these risk groups.
| 60825-1: | 62471-1: | Risk: |
|---|---|---|
| Class 1 (8h) | Risk Group 0 | Inherently safe |
| Class 1 | Risk Group 1 | Safe reasonably foreseeable conditions of operation |
| Class 1 M | ||
| Class 2 | Risk Group 2 | Safe based on aversion responses ( <0.25s exposure) |
| Class 2M | ||
| Class 3R | Risk Group 3 | Potentially hazardous for eye and skin. Safety measures are required |
| Class 3B | ||
| Class 4 |
While these efforts were well received and accepted in Japan and Europe, US regulators, namely the FDA, didn’t quite see it that way. In its ruling earlier in the year, it did not use the language that other agencies and LIPA provided and it referenced out of date EU regulations (the new one was approved but not issued). As a result, RGB and Laser Phosphor projectors are still considered laser devices and must obtain a variance for use in commercial application. That may be fine for a theater where an RGB laser will reside, but that also means a laser phosphor projector in a meeting room, symposium, or digital signage venue needs a variance from the FDA. That means limited access areas at a minimum.
The result is shown in the figure below. Under the IEC guideline, projectors with an output of 8,000 lumens and above are considered Risk Group 3, while the FDA guidelines set that limit at 3,000 lumens. That’s the difference between a specialized segment and the mainstream market.
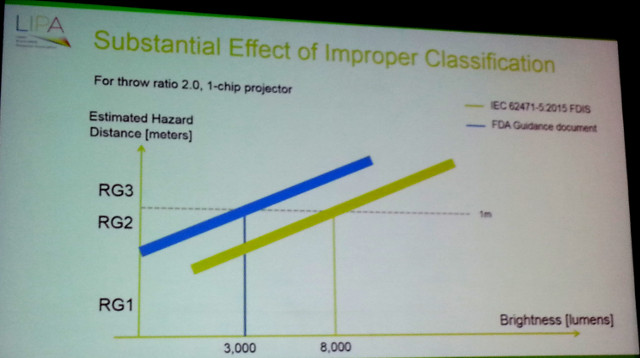
Such restrictions are clearly problematic for these markets. While projector makers could ignore the rules, they do so at their own risk, and many now appear to not be willing to take that chance, and that will have an impact on sales. The US projector market is 30-35% of the world market for projectors and laser-phosphor projectors are a hot category right now. As a result of the FDA ruling, it would not be surprising to see sales of projectors fall as the hot product carries too many risks and liabilities with it. – Chris Chinnock
Editor’s Note
The LIPA has sent us a response to the concerns raised in this article. LIPA Clarifies Its View on FDA/Laser Phosphor Issue – Bob