If you have been following the CES updates you have no doubt heard from LG about their newest OLED called “OLED EX” and their claim of “Deuterium-based stability.” So what in the world is deuterium and what does it have to do with OLED stability?

As a chemist and display enthusiast I was intrigued when our editor asked me to write about it. I have to be honest, it’s not something I had thought about much in the past, but I am always up for a challenge, especially when I can learn something new.
First, a chemistry lesson.
Hydrogen is known to exist as three isotopes (yes, dig deep for that general chemistry knowledge!). All three of these isotopes have one electron and one proton (charge = 0) but they contain different numbers of neutrons in the nucleus. Protium, Deuterium, and Tritium contain 0, 1, and 2 neutrons, but all are technically still considered to be hydrogen atoms.
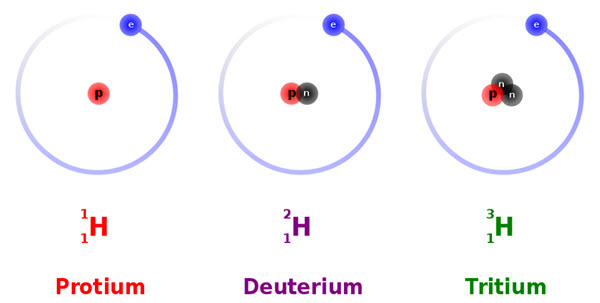 Source: https://commons.wikimedia.org/wiki/File:Hydrogen_Deuterium_Tritium_Nuclei_Schmatic-en.svg
Source: https://commons.wikimedia.org/wiki/File:Hydrogen_Deuterium_Tritium_Nuclei_Schmatic-en.svg
Of course, hydrogen atoms rarely exist alone, more commonly as H2, H2O or as an important component of organic molecules. You may have heard of “heavy water” before. Heavy water contains deuterium instead of protium and it is more dense than standard water. Enough so that frozen D2O actually sinks in water! I point this out simply to show that deuterium can result in some pretty interesting physical and chemical changes to materials.
 Water with simple floating ice cube H2O (left) and with “heavy” ice cube D2O which sinks due to higher density (right). Source
Water with simple floating ice cube H2O (left) and with “heavy” ice cube D2O which sinks due to higher density (right). Source
So how common is deuterium (and tritium)? Not very. Naturally occurring abundance of the three isotopes of hydrogen are as follows:
- 99.98% protium
- 0.016% deuterium
- <0.01% tritium
Tritium is, in fact, unstable (hence its role in hydrogen bombs) which is why it’s pretty rare. But deuterium is also quite low, even though it is stable. Only about one in every 6000+ hydrogen atoms contain that extra neutron which make it deuterium. This fact will come in handy later for trivia night (ok, unlikely, but it will come in handy later in this piece).
So what in world does deuterium have to do with OLEDs?
As it turns out, deuterium has been studied as an OLED component for over a decade now in both host and emitter materials. Even before this, deuterium has been used for decades to study what is called the “isotope effect” on chemical reactions. Basically, chemists can use deuterium atoms in place of hydrogen to study how this changes things like reaction kinetics (aka speed of a reaction). Molecules that normally contain a hydrogen atom bonded to carbon (C-H) will have faster kinetics than the same molecule where the H is replace by D (C-D). Essentially a C-D bond is harder to break compared to a C-H bond, so C-D will react slower (or require higher temperature)).
If you are a fellow chemist nerd like me who wants to geek-out on kinetic isotope effects, you can read more here.
Importantly, changing out H for D does not change electronic states, only kinetics, so things like emission wavelengths and energy levels should remain unchanged when dealing with OLED materials.
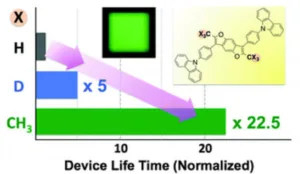
A shining (published) example of deuterium being used in and OLED devices from 2014 showed a dramatic 5x increase in lifetime when deuterium is used. In this example, deuterium was used in the host, and it was intentionally placed in a location on the molecule where the degradation mechanism was known to occur. Their data showed it would be even better if CH3 could replace H (>20x increase in lifetime) but that is not always realistic as the molecules would start to change at an electronic level.
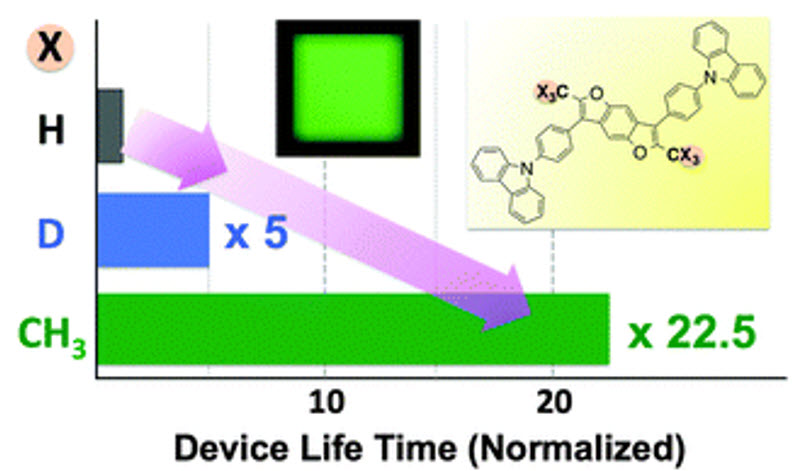 One example of how deuterium impacts OLED properties. Source: Chem. Commun. 2014
One example of how deuterium impacts OLED properties. Source: Chem. Commun. 2014
So essentially by using deuterium at a location that is normally the “weak link” in terms of stability, chemists can slow down the reactions that cause these molecules to break down, thereby enhancing operating lifetime.
At CES, LG claimed 30% improvement in lifetime with “deuterium-based stability.” Since LG, UDC, and others have already done so much optimization of their organic molecules for OLED I’m not surprised they can’t claim a 5X increase (the paper I referenced is from 2014 so there was more room for improvement at that time). But also keep in mind this enhancement from deuterium may allow for the OLED to be run hotter (brighter) with less risk of burn in, reducing a major perceived risk for consumers while bolstering a historical weakness of OLED compared to LCDs (brightness).
So, why don’t chemists just change out H for D in all the OLED emitters, hosts, etc.? Trust me, we all wish it were that easy. Remember the trivia knowledge from earlier that only 1 in every 6000+ hydrogen atoms are deuterium? Herein lies the challenge. Isolating (or enriching) deuterium-containing molecules is hard and expensive work. And it’s not always perfect. So simply getting the precursors to make the deuterated molecules can be a challenge. And if you can get it, you better believe you will pay for it. For example, a liter of 99.9% D2O from Sigma Aldrich will run you north of $1,300 (as of January 2022). A liter of deionized water from the same source: ~$10/L (still a rip-off but you get the idea).
Clearly LG or their suppliers have figured out a cost-effective way to implement deuterium. And perhaps it has been happening for years but they are only now talking about it? Ultimately LG choose to use deuterium in their marketing probably because OLED’s have always had to answer the questions of burn in, lifetime, and brightness (when compared to LCD, and now QD-OLED too). Now LG can at least point to something concrete that has been done to mitigate this effect. Only time will tell if burn-in remains an issue or not. (PP)
Peter Palomaki is the owner and chief scientist at Palomaki Consulting, a firm specializing in helping companies solve big problems at the nanoscale. His utilizes his expertise in quantum dots and materials chemistry to solve challenging problems with clients large and small.